In this context, the Eutectic Effect is the result of silver combining with copper to form a new alloy, which has a lower melting temperature than either of the two original metals. This used to be considered an error, but is now made into a technique. Averill B. Shepps wrote the project in the book, which tells how to somewhat control when the new alloy is produced. This control is based on enamels with Chromium in them (these inhibit the effect). Cullen Hackler says chrome, nickel and iron will inhibit the effect.
TOC
- Enamels To Inhibit The Eutectic Effect
- Copper Foil and The Eutectic Effect
- Stenciling For a Eutectic Shape
Enamels To Inhibit The Eutectic Effect
The project in the book says that some of the greens and maybe some browns have Chromium, but it is left to the reader to test which work for this purpose. I tested Thompson Enamel enamels, with help by Jean Tudor and Cullen Hackler. Cullen Hackler also suggests trying the greens in the higher numbered 2300s. Here are the results of my testing:
- Colors that seem to inhibit the Eutectic Efffect
- Gem green (2325)
- Peacock Green (2335)
- Glass Green (2340)
- Oil Gray (2915) (probably contains nickel)
- Colors that do not inhibit the Eutectic Effect
- Chestnut Brown (2190)
- Lime (2230)
- Peppermint Green (2310)
- Grass Green (2350)
- Colors still to try
(there are many more I'm sure)
- Elan Gray (2910)
- Spring Green (2320)
- Sea Green (2420)
if you try any others of these, I would appreciate if you contact me to discuss your results so I can post them here.
Copper Foil And the Eutectic Effect
Cullen Hackler suggested trying to use copper foil (26 ga) to control the shape of the Eutectic Effect. I started playing with this and was partically successful. I think the hard part is determining how much silver to put on the copper foil to ensure coverage.
Foil on pre-enameled surface I first tried cutting a shape out of foil and adhering it a pre-enameled copper square. I sifted an inhibiting enamel as a light base, laid down the foil, covered that will a non-inhibiting enamel and added the silver and fired. As you can see it worked but didn't give em what I wanted. |
|
 |
 |
Foil on base coat before firing Then I tried cutting a shape and adhering it to the 1st layer of enamel base coat, which was an inhibiting enamel color. I sgraffitoed two places for my "flowers". In one I put a wire on the 1st firing and not on the other to see if there woudl be a difference. After firing I pickled the piece to get rid of the firescale. For the second firing, I added non-hibiting colors to the bare copper and added my silver and fired. This looked very promising and did work some, but possibly because of the shape I used, the background ran into my shape and I wasn't totally successful, but it also could be that I didn't use enough silver. Also, it seemed that the silver that were flat pieces didn't work so they probably were not touching the copper directly as recommended in the book's project. One thing I think I did wrong - a big enameling no no - I forgot to let the Klyr-fire dry before I fired so some of the silver moved out of place! |
|
 |
|
 |
 |
If you try this and get successful, please le me know! Also, if you can figure out a way of knowing if you put on enough silver, let me know!
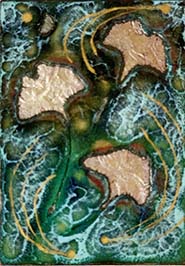
Stenciling For A Eutectic Shape
The project in the book uses stenciing to separate what is and is not part of the Eutectic Effect. The stencil shapes used were rectangles. I wanted to try a specific shape. Again, it worked, but I didn't use enough silver to complete the shape - at least that is what I think I did wrong. Here is what I got.
 |
 |
After this I tried doing it on a real piece using the shapes of Ginkgo leaves for the stencils. I was happy with the outcome. This piece is an Artist Trading Card (ATC), which I find to be a great size to work on (because I'm used to working under 2"). To polish the Eutectic spaces, I used an 80 grit 3M Radial Bristle Disc - cleans it right up.